Quaterly compendium of biosciences industry perspectives, Q3 September 2018
CRISPR Patent fight
In 2012, a research team led by Jennifer Doudna of University of California Berkeley and Emmanuelle Charpentier, a french microbiologist of the Max Planck Institute in Germany, published the first article (Science, August 2012) that described how CRISPR-Cas9 can make precise alterations in the DNA in eukaryotic cells. This article is considered as a dramatic contribution with tremendous industrial potential. Since then, it has paved the way of a huge research effort enabling a new way to treat diseases, as reported in a recent landscape analysis (Clarivate Analytics, May 2018).
Thema concept map of major concepts and subject matters from 4,450 DWPI analyzed Patent Families related to CRISPR. Clarivate landscape report
Another team led by Feng Zhang from the Broad Institute reported the use of CRISPR to cut DNA in human cells (Science, February 2012). In April 2014, Broad received the first patent of a series for the mammalian cell use of CRISPR, after a fast-tracked review by USPTO. UC which filed a patent before Broad, were granted two patents in June 2014, and sued in 2016 the Broad before the USPTO for patent infringement. In February 2017, the Patent Trial and Appeal Board (PTAB) ruled in favor of Broad (Science, April 2018), against patent interference from January 2016 (Science, February 2017) argued by UC. Although UC was able to show that some of the techniques used to transfer systems into eukaryotes were known to the person ordinary skilled in the art, PTAB considered the one skilled in the art would not have reasonably expected a CRISPR-Cas9 system to be successful in a eukaryotic environment, because UC had only published results in prokaryotic cells and in vitro.
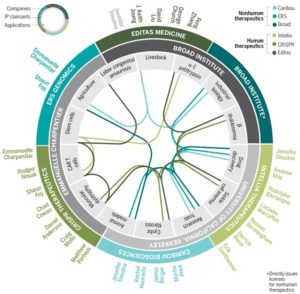
The CRISPR market Pie. G. Grullón/Science.
In September 3018, the U.S Court of Appeals for the Federal Circuits (CAFC, September 2018) ruled that the Broad and UC were patenting sufficiently distinct material and both patent will stand, meaning that Broad will continue to hold intellectual property for the use of the CRISPR gene editing in eukaryotes, separately from UC’s invention of the use of CRISPR-Cas9 in all environments including plant and animal cells. Hence, CRISPR-Cas9 patent claims owned by UC and Broad respectively remain unsolved (Pswire, September 2018). In 2018, EPO revoked Broad’s EP2771468 patent, and found all claims of the patent to be invalid, due to a procedural defect and was challenged on its competency to rule on priority (Article 87 of the European Patent Convention) as compared to US laws for inventorship (Sciencemag, January 2018).
Petition to the Supreme Court concerning PCSK9 patents
The economic battle is between Alirocumab and Evolocumab for the treatment of cardiovascular risk. After prosecution started in March 2016 at the district court of Delaware, Sanofi received a permanent injunction to withdraw Alirocumab from the market (dossier in appeal January 13, 2017). This decision has been reversed by the Court of Appeals (Federal circuit result for appeal October 5, 2017) due to improper instruction of the jury concerning written description and improper issue of the permanent injunction. In fact the district court of Delaware stated that antigen description was sufficient to enable an antibody. This is not always the case. The court of appeal stated regarding the Noelle’s case[1] and the PTO Guidelines (Guidelines, Page 60), the conclusion that instead of “analogizing the antibody-antigen relationship to a key in a lock,” it was more apt to analogize it to a lock and “a ring with a million keys on it.” Antigen enablement for a claim of an antibody raises also the question of the patentability of ‘nature’, as antigen is a mechanism of ‘nature’ not ‘manufacture’ as stated in the Myriad case[2]. The district court also improperly issued the permanent injunction because it was in contradiction with the public interest. Therefore Sanofi recovered its right to propose Alirocumab for patient treatment. However, this summer, Amgen came back and reloaded the litigation to advocate for enablement of an antibody given the antigen. The company filed a petition to the Supreme Court (petition to the Supreme Court July 23, 2018).
Le Belvédère, September 24th 2018
[1] Noelle v. Lederman, 355 F.3d 1343 (Fed. Cir. 2004)
[2] Association for Molecular Pathology v. Myriad Genetics, Inc., 133 S. Ct. 2107(2013)